COMSOL has the best multiphysical simulation capabilities in my experience. Technical support from Elisa at TECHNIC as well as the engineers at COMSOL has been great.
COMSOL is an important part of our research in plasma physics. We use it in the design of plasma systems and it helps us to obtain a greater understanding of the underlying physics. We have always valued the quick support from TECHNIC and COMSOL and it has been a pleasure to work with them.
Comsol has become a valuable part of our design and decision making process. The exceptional flexibility and access to the physics and solvers in Comsol has allowed us to have deeper understanding on thermomechanical solutions. Technic and Comsol have always been quick and helpful to resolve any issues and provide helpful advice on their products.
At Scion we use COMSOL Multiphysics to understand energy processes, such as the interplay of non-linear solid mechanics and heat & mass transfer during biomass compaction, to design new or more efficient processes.
We use COMSOL Multiphysics to design the customised muffler. With it, we can simulate the insertion loss at different spectrum with different muffler designs.
Modelling batteries requires different levels of detail depending of the purpose of the simulations. The Battery Design Module is an add-on to the COMSOL Multiphysics® software that encompasses descriptions over a large range of scales, from the detailed structures in the battery's porous electrode to the battery pack scale including thermal management systems.
The descriptions involve physics phenomena such as transport of charged and neutral species, charge balances, chemical and electrochemical reactions, Joule heating and thermal effects due to electrochemical reactions, heat transfer, fluid flow, as well as other physical phenomena important for the understanding of a battery system. For well-known and verified systems, lumped models are available and may be physics-based or based on equivalent circuits.
Note that the name of this product changed from the Batteries & Fuel Cells Module to the Battery Design Module with the release of version 5.6, while retaining all functionality. For users that model fuel cells and electrolysers, a new Fuel Cells & Electrolysers Module is available.
The lithium-ion battery is the most popular battery for portable applications due to its high power and energy density. The Battery Design Module features state-of-the-art models for lithium-Ion batteries. The so-called Newman model is predefined in the module with the latest findings in scientific literature. For example, different mechanisms for ageing have been built-in, such as growth of the SEI, metal plating, short-circuiting, and electrolyte degradation. These high-fidelity models are available for 1D, 2D, and full 3D modelling, with an additional pseudo-dimension for modelling the intercalation of lithium in the electrode particles.
In addition to modelling the electrochemical reactions, when combining with heat transfer, a full energy balance is added. You are also able to account for the structural stresses and strains caused by the expansion and contraction from lithium intercalation, when combined with the Structural Mechanics Module.
For the latest trend in battery modelling, the module also includes functionality for heterogenous models, where the detailed structure of the porous electrodes and the pore electrolyte can be modelled for a representative unit cell of a battery. Such models may be used for a deeper understanding of the impact of the microstructure of a battery.
The Battery Design Module contains one of the most advanced battery models for simulating lead–acid batteries. The software includes the dependent variables for the ionic potential in the electrolyte (both separator and pore electrolyte), the electric potential in the solid electrodes (and current collectors/feeders), the composition of the electrolyte, and the porosity of the electrodes. The module also contains a thermodynamic and kinetic parameters database for the lead–acid battery.
A typical use is to study the effect of design parameters on the performance of the battery, such as thickness and geometry of the electrodes and separators, the geometry of the current collectors and feeders, the porosity of the electrodes, the geometry and composition of the separator, to mention a few.
The studies that can be run include full transient studies, including the effect of double-layer capacitance, as well as impedance spectroscopy studies in the frequency domain.
The workhorse of the Battery Design Module is the detailed model of the battery unit cells with positive electrode, negative electrode, and separator. In the electrodes, the pore electrolyte is in contact with the electrolyte in the separator.
The porous structure in the electrodes is homogenised, meaning that the pore electrolyte and the solid electrode material are present everywhere in space, and a volume fraction determines the respective properties of the phases. The transport equations and the electrochemical and chemical reactions are treated with so-called porous electrode theory as devised by Newman in the book Electrochemical Systems.
With the generic description of porous electrodes, you can define any number of competing reactions in an electrode and also couple this to an electrolyte of an arbitrary composition. For example, a tutorial model of the vanadium battery is included in the module's Application Library.
The pore electrolyte and the electrolyte in the separator can be described, for any composition, with the theory for concentrated electrolytes, dilute electrolytes (Nernst–Planck equations), and supporting electrolytes.
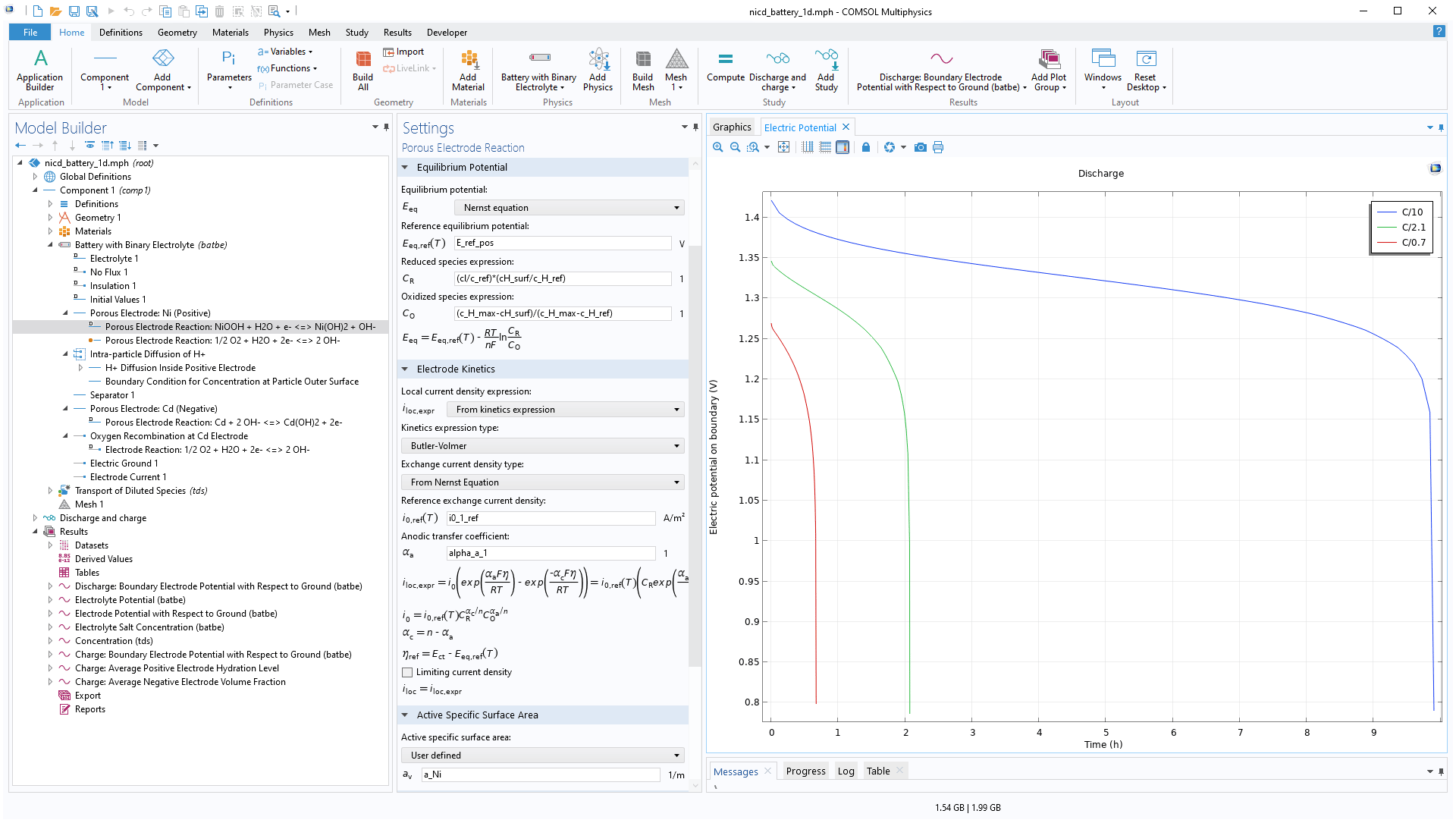
A specific version for batteries with binary electrolytes is available as predefined functionality. You can use this to model NiMH and NiCd batteries, and allows intercalating materials in the solid phase, such as hydrogen, for example.
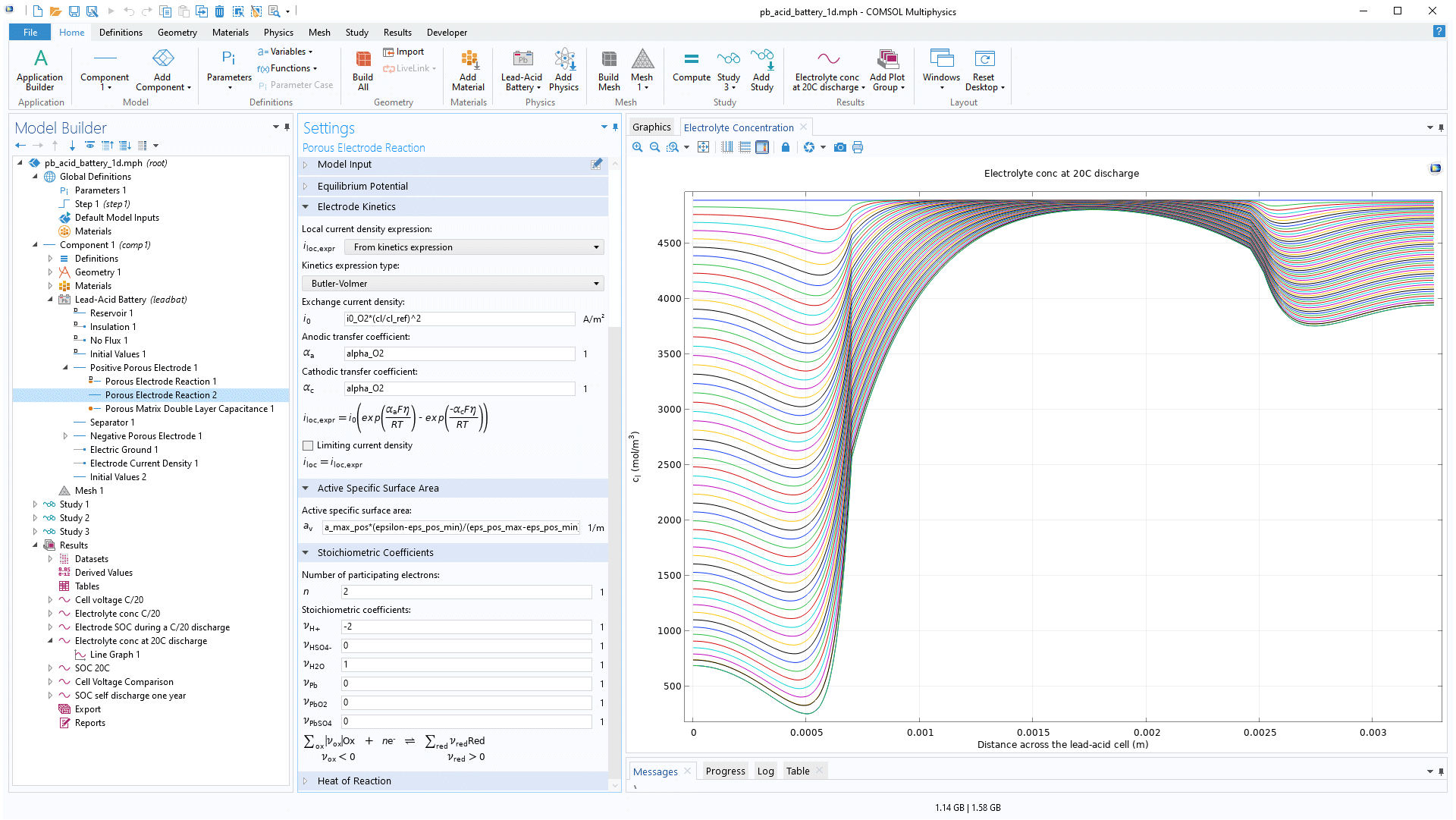
The particles in porous battery electrodes can either be solid (Li-ion electrode) or porous (lead–acid, NiCd).
In the case of solid particles, the porosity in the electrode is found between the packed particles. However, transport and reactions may occur in the solid particles for small atoms such as hydrogen and lithium atoms. These intercalating species are modelled with a separate diffusion-reaction equation defined along the radius of the solid particles. The flux of the intercalating species is coupled at the surface of the particles with the species that are transported in the pore electrolyte between the particles. The intercalation species and reactions are predefined for Li-ion batteries, however, you can use the same functionality to model intercalation of hydrogen in, for example, NiMH batteries.
In the case of porous particles, a bimodal pore structure is obtained: a macroporous structure between the packed particles and a microporous structure inside the particles. The reaction-diffusion equations in the porous particles are defined in a similar fashion as for the intercalation of species in solid particles. This is exemplified in the NiCd tutorial model included in the module's Application Library.
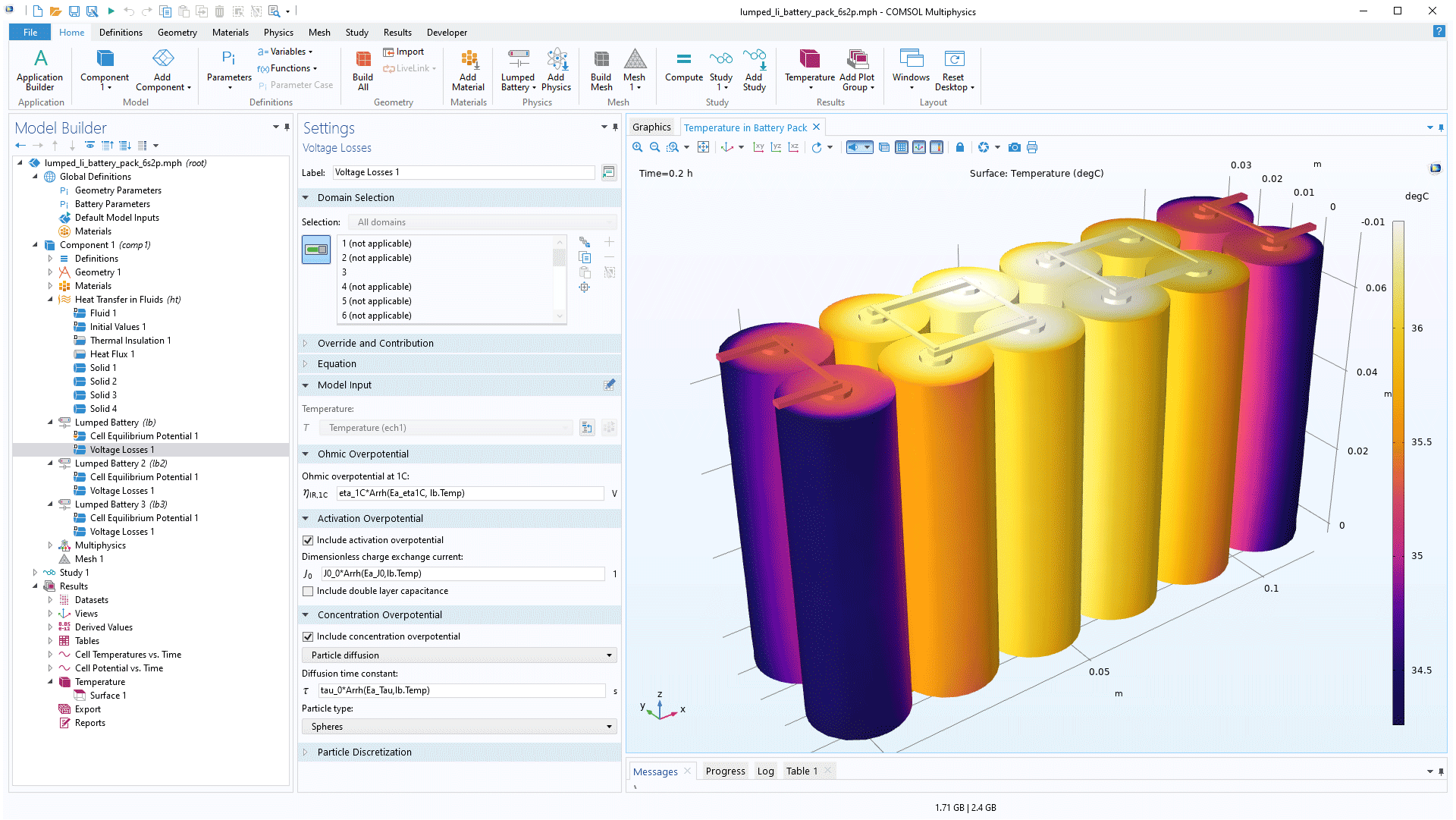
The thermal analysis of battery packs can be time-consuming if we use full 3D models for the electrochemistry. An alternative is to use validated lumped (simplified) models for each battery in a pack. Once validated, the lumped models may give an excellent accuracy within a particular (maybe limited) range of operation.
The Battery Design Module contains lumped models that are physics-based and solve the electrochemical equations in 1D plus a pseudo-dimension (particle dimension); 0D plus a pseudo-dimension; and pure 0D models, such as equivalent circuit models, for example.
The multicomponent model may contain the full range of fidelity, from the detailed 3D models to the lumped 0D models. These models are incorporated as separate components in a multicomponent model file. It is therefore easy to alternate between lumped models and use detailed models when the lumped models need to be updated and validated for a new range of operation.
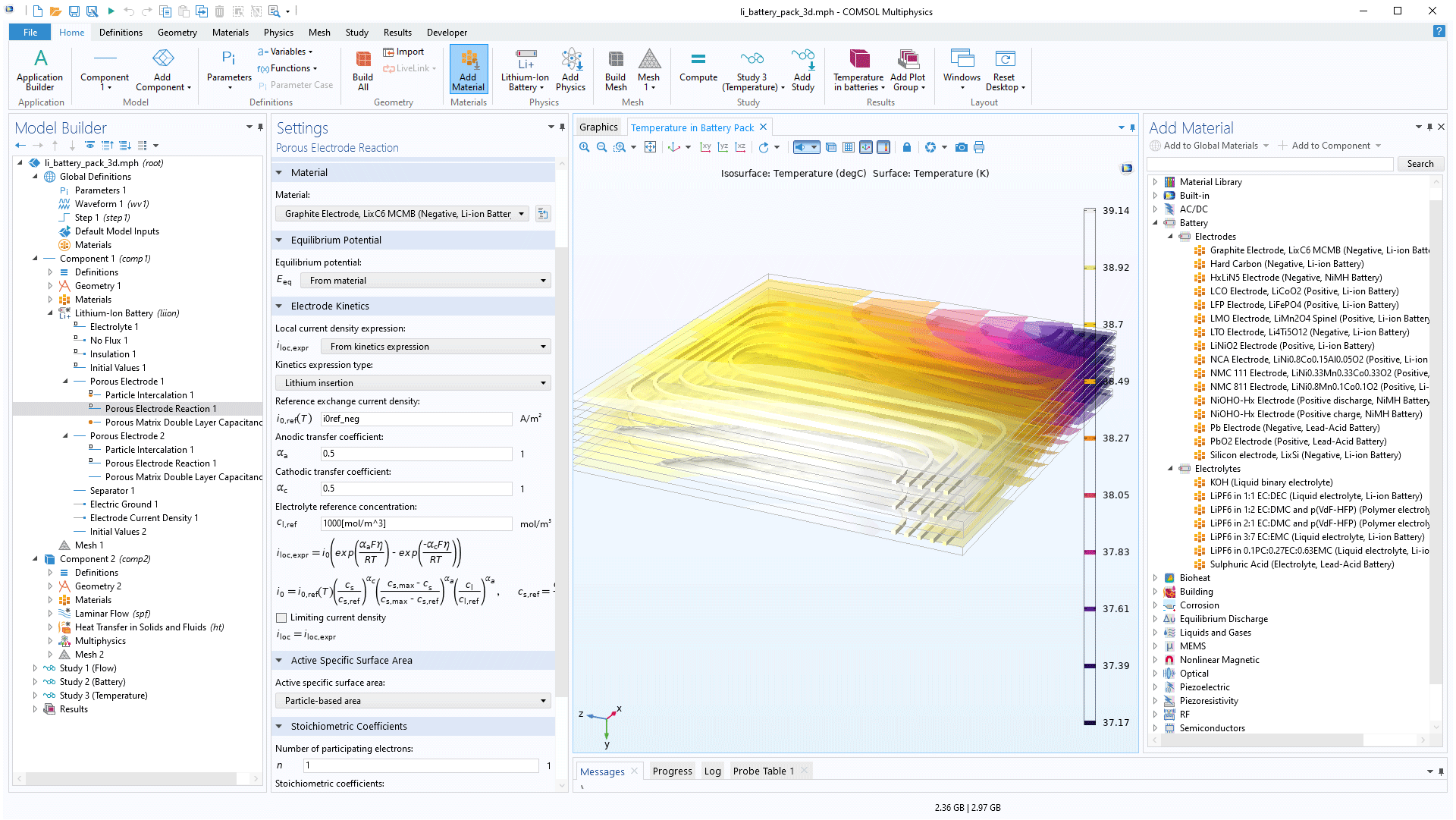
One of the more time-consuming and error prone steps in the modelling of battery systems is to gather input data and to use it consistently. For example, it is important that the positive and negative electrodes are defined in the same reference systems. The equilibrium electrode (half-cell) potentials have to be measured or calibrated to the same reference electrodes, electrolytes, and temperatures before they are incorporated in the same battery system model.
The battery material database included in the module contains entries for a number of common electrodes and electrolytes, substantially reducing the amount of work needed for creating new battery models.
In order to fully evaluate whether or not the COMSOL Multiphysics® software will meet your requirements, you need to contact us. By talking to one of our sales representatives, you will get personalised recommendations and fully documented examples to help you get the most out of your evaluation and guide you to choose the best license option to suit your needs.
Fill in your contact details and any specific comments or questions, and submit. You will receive a response from a sales representative within one business day.